Big Pharma Portfolio Strategy isn’t about big volume = growth anymore
After 20+ years of strong but selective diversification, Big Pharma Portfolio Strategy and Management isn’t about big volume = growth anymore but about focus, collaboration and coordination. Active de-risking of portfolios becomes more important, as well as strategically thinking about the role and position of the Portfolio Strategy and Management Office or various portfolio teams within the wider organisation.
ARTICLES
Irene Petre
12/4/20255 min read


R&D spend remains stubbornly high, despite AI making inroads
For over two decades Big Pharma grew big and bigger by developing large portfolios across many therapeutic areas and indications, large companies featured tens or hundreds of commercialised products but also hundreds of drug candidates in the pipeline (which was meant to ensure at least a few successful ones would become blockbusters on the market). Curating large complex portfolios became the norm, although this diversification trend was somehow more selective compared to the 60s and 70s.
But it seems that over the last year or so, volume doesn’t equal revenue growth that easily anymore and complexity isn’t (that often) beneficial. Focussing the efforts, time and investment on just one or a handful of key products seems to be now the winning strategy.
Boards are now increasingly asking: “Which assets actually deserve our investment, manufacturing capacity and focussed attention?”
Overall R&D and manufacturing unit costs remain high (although in the last couple of years productivity showed some signs of improvement) and in fact have increased over the past decade. Despite AI promises to slash time and costs, this hasn’t so far materialised in significant reductions and AI remains more of an enabler, rather than a net cost reduction factor.
Total R&D spend and per‑asset lifecycle costs remain in the multi‑billion dollar range for a successful new molecular entity finds IQVIA (1), whilst according to Fierce Biotech and Deloitte the average full cycle development costs are around USD 2- 3bn per approved asset (2, 3) in 2023 and 2024.
This is not surprising given that an increased number of Pharma companies develop and commercialise more and more biologics (which carry significantly higher costs), labour costs have by and large increased in most countries and regulatory compliance has become more complex and costly, especially in Europe.
Whilst it is true that AI and advanced analytics promise productivity and cost gains (from target discovery to trial design, patient recruitment and actual manufacturing), its adoption is uneven across a large organisation and requires upfront investment in data, platforms and governance, thus creating new costs (for data engineering, validation, regulatory work).
Some sources:
Pharma companies are increasingly focussing on which key assets, therapeutic areas and platforms truly deserve attention
Given these persistent high costs and increased complexity of the drug development process, it becomes more difficult to continue to maintain very large portfolios and the market seems to reward players like Eli Lily who have managed to leverage commercial scale from one key indication – the GLP‑1 franchise (Mounjaro/Zepbound) and its metabolic blockbusters. Its key analytics seem to lie in manufacturing capacity, pricing/payer risk and rapid global roll‑out scenarios, rather than broad early‑stage diversification.
Another Big Pharma, AstraZeneca has largely existed some TAs such as Neuroscience earlier this year, closing its dedicated research group in order to focus on "core" areas like Oncology (its main ambition), Cardiovascular, Respiratory and Immunology. The firm discontinued its Alzheimer and pain management (migraine) programs and it may also discontinue its monoclonal antibody against Parkinson's disease, after some bad Phase 2 study results. This is not totally surprising - Neuroscience is a particularly difficult and risky area for drug R&D and many large firms have existed in recent years, including Pfizer and Amgen.
Key challenges for Pharma keep coming from both AI and tech developments (which then trigger more digital and compliance costs) but also regulatory complexity: whilst Europe seems to lead in this respect, with the shift in administration – the US is also changing its policies at a rapid pace. For example the policy shift towards the MPN (Most Preferred Nation) is likely to impact not just pricing and Market Access, but many other strategic decisions and functions within Pharma, including Portfolio Planning, Management and Strategy. These teams must shift from periodic, finance-driven analytics to continuous, focussed, strategic and multi-dimensional decision engines that encompass clinical probability, HTA and reimbursement risk, RWE readiness, manufacturing constraints and - now with policies such as MPN (that if implemented, will likely be adopted in other countries too) – global pricing scenarios.
So active de-risking of portfolios emerge as truly important as well as the ability to focus portfolios on key TAs (Therapeutic Areas), indications (4) and technology platforms. Focusing on technology platforms (such as modality platforms - mRNA, cell and gene therapy etc., radioligand and targeted delivery platforms, data and RWE platforms, manufacturing platforms etc.) means concentrating on how drugs are made, delivered and demonstrated, not just what disease they treat. Platforms create repeatable capabilities, lower marginal cost per asset, and accelerate learning across programs.
Towards a Centralised Portfolio Office?
Therefore a key strategic shift especially for large Pharma – that are still typically siloed in their processes and mentality – becomes the awareness and ability to design and implement a Centralised Portfolio Office that owns the data and the scenario engines and works across business units and functions, collaboratively. The ability to integrate regulatory and policy intelligence and portfolio de-risking strategies into these Centralised Portfolio Teams will represent key advantages – as policy shifts become more pervasive (e.g. EU AI Act, MDR, US IRA, MPN, Medicare negotiations etc.) and far reaching. Of course this is easier said than done and top executives in Pharma need to be among the first to understand this need for change and actively support it.
A centralised portfolio office can help focus the Pharma (and MedTech) portfolio and decision-making process around it. Focusing the portfolio is important because this concentrates scarce capital, reduces managerial complexity and allows companies to make more significant investments per fewer assets, improving PoS (probability of success) due to better trial design, targeted RWE and faster regulatory/HTA readiness - thus potentially reducing time to market as well.




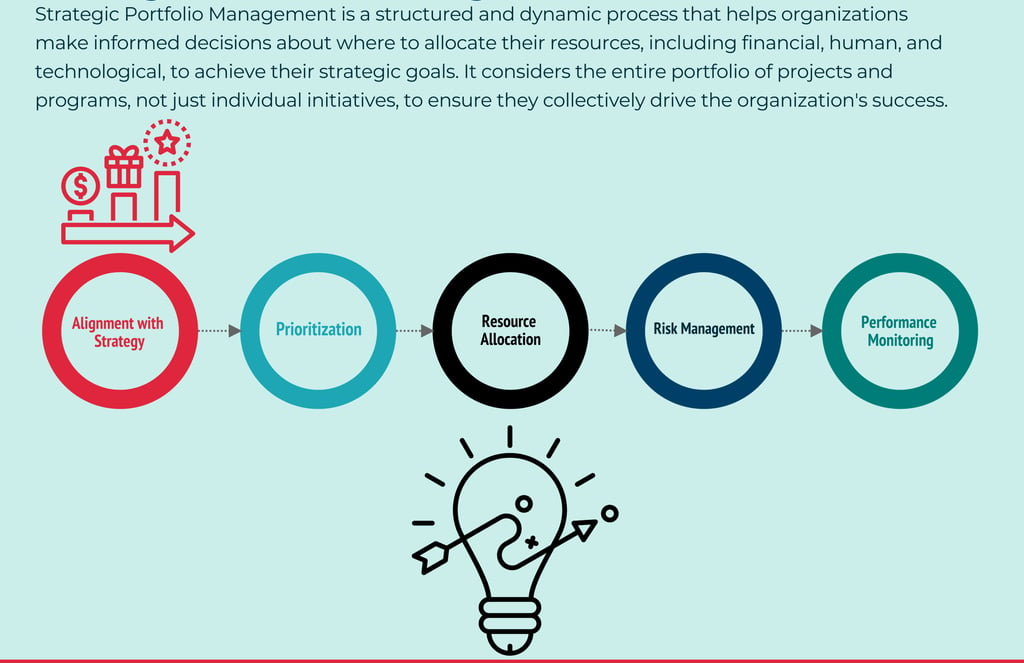
There are many frameworks and theories for what Portfolio Management, Planning or Strategy should include and exclude...
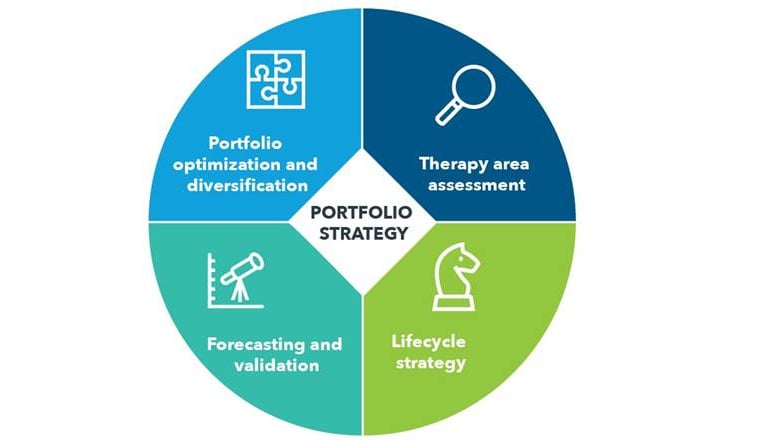
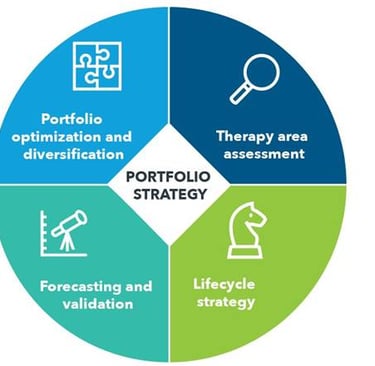
IGEA Healthcare
Strategic Advisory for Life Sciences
Switzerland, UK, Italy
contact@igeahealthcare.com
© 2025. All rights reserved.
